Scaling to New Heights: Robust Scale Up from Shake Flask to Commercial AAV Manufacturing for All Patient Populations
This article was first published in Fierce Biotech.
Author:

Cliff Aquino
Senior Manager, Technical Sales and Scientific Advisory Forge Biologics
Data Contributor:
 Mason Bonitz
Mason Bonitz
Scientist, Process Development, Forge Biologics
Introduction
Genetic therapies utilizing adeno-associated viral (AAV) vectors have evolved in recent years from proof of concept to successful treatment options for patients with previously difficult to treat diseases. AAV vectors, known for their ability to safely and efficiently deliver and introduce a correct copy of a gene into patient cells, have become essential for treating many devastating genetic diseases. However, to be successful for multiple and larger patient populations, these groundbreaking therapies must overcome the critical challenge of manufacturing at a scale that meets the growing global need.
While there has been much progress in bringing these therapies forward to the market, they still require immense resources to develop and manufacture a quality drug product at a commercial scale. Forge Biologics, an end-to-end genetic medicines contract development and manufacturing organization, has put a strategic emphasis on scaling up processes so that developers working on larger indications or patient populations can efficiently manufacture at the necessary scales. Scaling to 1,000L or industry-leading 5,000L in a single-use bioreactor provides economies of scale to gene therapy developers, reducing costs of goods (COGS) while still providing high-quality, safe, and effective drug products.
Recognizing the capacity and technical gaps in the current market needed to deliver treatments to patients, Forge has streamlined the AAV production process. By establishing a bespoke platform approach to effectively bridge the gap between small-scale laboratory experiments and commercial-scale manufacturing, Forge’s platform accelerates gene therapy programs to and through the clinic to reach patients faster. Additionally, the same process, cell line, facility, and analytics can be utilized starting at the research-grade production level. This helps reduce any need for bridging studies as manufacturing moves through preclinical, clinical, and commercial development planning.
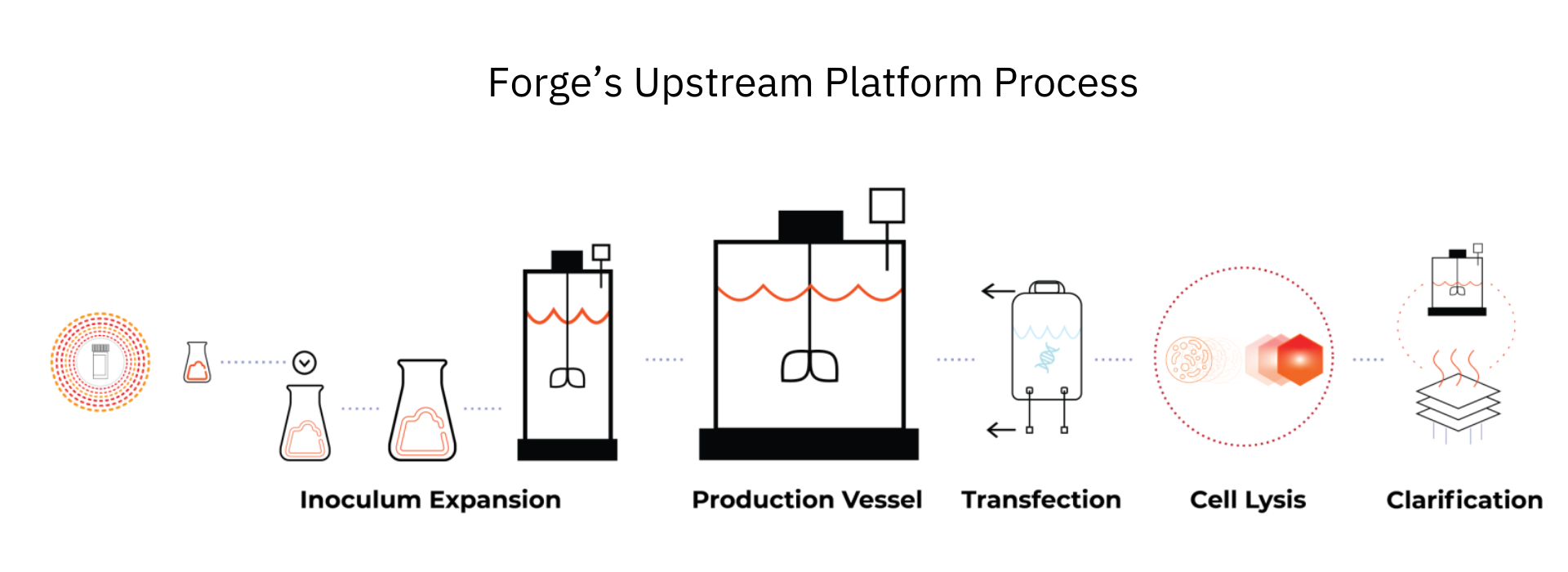
Manufacturing Platform Selection
The most widely accepted production platform for AAV vectors utilizes the HEK293 cell line.1 HEK293 cells can be grown successfully in adherent or suspension cultures. While effective for smaller scales, adherent cell cultures are currently limited to a scale out model for expansion. This presents logistical concerns when larger culture volumes are needed for higher yields required for commercial-stage large patient populations.
Recognizing the inherent limitations of adherent cell lines, Forge developed an in-house suspension-adapted HEK293 cell line known as Ignition Cells. This strategy of focusing on suspension allows Forge to scale up to commercial-scale bioreactors and overcome the limitations of adherent cells, as well as the ability to scale out to increase capacity and help meet the vast and growing AAV gene therapy demand.

Overcoming Challenges of Scaling AAV Vector Upstream Manufacturing Processes
Scaling up bioreactors for suspension AAV manufacturing requires a comprehensive understanding of engineering, physics, the biological properties of mammalian cells, and viral stability in culture. As processing volumes expand, bioreactor designs and associated parameters become increasingly intricate, necessitating a thorough assessment of scale-dependent factors. With numerous variables, attempting to scale without specialized expertise can be challenging and costly. Key considerations when scaling bioreactors include Mixing, Aeration and Mass Transfer, System Control Loops, and Logistics.
-1.png?width=654&height=436&name=MicrosoftTeams-image%20(169)-1.png) Mixing
Mixing
Adequate mixing in the bioreactor ensures that cells, nutrients, and other reagents are uniformly distributed to support consistent growth conditions and optimal production yields. As we scale up, the dynamics of mixing change, potentially creating areas of varied temperature and concentration profiles that can be detrimental to production.
This challenge is compounded by the generation of shear stress from the mechanical forces of the impeller(s) and foam production, which can hinder gas exchange and disrupt cell growth. Although reagents can be introduced to mitigate these effects, excessive additives can also adversely affect the process. Achieving optimal mixing in bioreactors is imperative and requires a comprehensive strategy to effectively address these challenges and ensure the success of each batch.
Aeration and Mass Transfer
Through the process of aeration, optimal dissolved oxygen (DO) levels are maintained in the bioreactor, which is crucial for cell growth and vector productivity. The two main mechanisms for aeration are through direct sparging of gases into the cell culture medium, or gas overlay across the surface of the medium, often supporting the removal of off-gases. As cells grow and with increased processing scales, the Oxygen Uptake Rate (OUR) increases. To avoid the Oxygen Transfer Rate (OTR) being the metabolic rate-limiting factor, the kLa (Mass Transfer) coefficient, measuring the conversion of oxygen from a gaseous to a liquid state, must be interrogated. Since kLa is dependent on the diffusion coefficent of the liquid medium, bubble size distribution, and dispersion across the bioreactor, the most common approach is altering the sparging strategy and agitation.2 With the goal of ensuring each cell gets adequate oxygen, it is important to avoid increasing gas flow and agitation to the points of shear and oxidative stress.
System Control Loops
The fine-tuning of process parameter control systems is a meticulous, iterative process that requires expertise in engineering and cell biology. Whether employing a closed feedback loop, cascade, or other control systems, the adjustment of Proportional - Integral - Derivative (PID) controllers – or subsets – becomes necessary as the scale of processing increases. PID controllers serve as crucial instruments for control systems, influencing the bioreactor’s response when a process parameter deviates from the set point or falls outside the established tolerance zone, known as dead band. Improper PID settings can cause the system to react too quickly or aggressively, leading to pronounced oscillations in process values. Conversely, settings can result in sluggish responses and allow values to deviate from the acceptable range, thus increasing process variability.3 The right balance of Proportional, Integral, and Derivative values is necessary to achieve stable and responsive control with minimal oscillations for optimal control performance.
Logistics
A critical and often underestimated challenge of scaling is adjusting methods that are straightforward at benchtop scale but become complex as volumes increase. For instance, simple additions to the bioreactor can require innovative engineering solutions, especially for time-sensitive operations. A primary example is inoculation, the addition of cells to batched media. Larger bioreactors require substantial inoculum volumes to initiate the batch. Given that HEK293 cells are sensitive to shear, developing an effective transfer strategy requires careful consideration, such as exploring various pump systems and evaluating flow rate and transfer time to identify proven acceptance ranges (PARs) to maintain cell health.
Equally critical is the process of transient transfection, which relies on the precise complexation of three plasmids: pGOI, pHelper, and pRep/Cap, with a cationic polymer like polyethyleneimine (PEI). While optimizing transfection at a smaller scale is necessary, scaling up introduces a host of variables–from the operating equipment and the timing of complexation to the method of transfection cocktail delivery. Each of these variables demands investigation and engineering controls. The goal is to ensure consistent, efficient transfection, crucial for high-yield AAV processes.
-1.png?width=787&height=525&name=MicrosoftTeams-image%20(166)-1.png)
Scaling To New Heights at Forge
The experts at Forge have devoted significant time and effort to overcoming scaling challenges to support AAV manufacturing from 250mL to 1,000L. Forge has established a platform process utilizing Ignition Cells that drives consistency informed by the following:
-
Incorporation of bioreactor design metrics to predict optimal scaling parameters
-
Extensive kLa studies to optimize sparge profiles and agitation rates
-
Fine-tuned PID controllers at various bioreactor scales to maintain robust engineering control of key process parameters
-
Pioneered innovative processing methods for time-sensitive operations at scale, overcoming the logistical challenges between small-scale and commercial-scale
As our data supports, Forge’s platform process achieves comparable vector productivity across scales, as seen in Figure 1.
Figure 1: Consistent Yields from 1L to 500L
.jpg?width=800&height=466&name=Consistent%20Yields%20from%201L%20to%20500L%20(No%20Borders).jpg)
Figure 1: Consistent Yield from 1L to 500L Scale. Platform development results where Ignition Cells were cultured in F17 media up to production-scale (1L SF, 5L, 50L, 250L, 500L SUB). Once cells reached the target VCD, the culture underwent triple plasmid transfection using AAV9 Rep/Cap, eGFP Transgene, and pEMBRTM Ad Helper Plasmids. The AAV9.eGFP product was then harvested on day 4 through lysis, nuclease treatment, and depth filtration. Samples were taken and submitted for GOI-specific ddPCR titer analysis. All samples were analyzed for titer and absence of matrix inhibition.
Ensuring consistency demands numerous development and characterization experiments of the platform. From the onset, Forge’s process development team emphasized scalability, tailoring the platform to address scale-dependent factors. For example, evaluating power per unit volume (W/m3) can be tested to determine the impact of mixing on cell growth, product yield, and product quality.
During the agitation characterization study, shown in Figure 2, growth profiles of the Ignition Cells were examined in 5L SUBs at preset agitation rates varied based on power per unit volume. Power per unit volume is one of several metrics, such as tip speed and KLa3, that can be used to maintain consistency during scale-up. The characterization can identify optimal agitation rates and/or inform PARs if dynamic agitation is required.
Figure 2: Impact of Bioreactor Agitation on Viable Cell Density Over Time
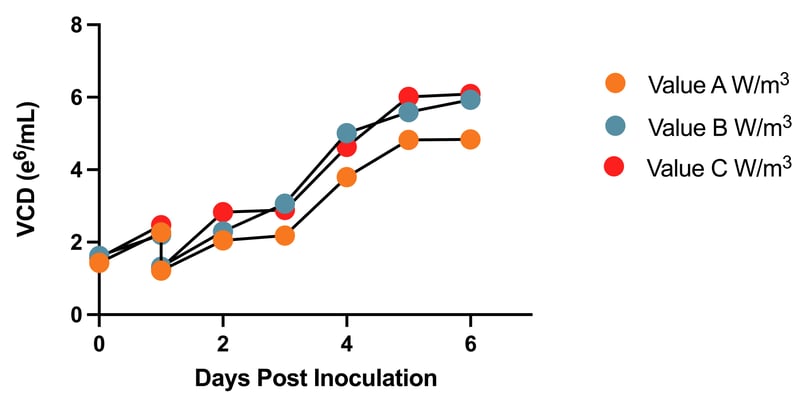
Figure 2: Impact of Bioreactor Agitation on Viable Cell Density Over Time. Cell growth profiles were compared to assess the impact of bioreactor agitation. Ignition Cells were seeded in 5L bioreactors and diluted to full working volume one day following inoculation. Each bioreactor was set to an agitation rate in RPMs based on the power per unit volume (W/m3). Samples were taken every 24 hours for six days to measure the viable cell density.
Transfection method optimization for the platform process, shown in Figure 3, was a necessary focus area as transfection is essential to batch productivity and is a known source of variability in the AAV manufacturing process.4 The research-based approach relied on the operator introducing all compo-nents into a single container (ex., Flask) within a biosafety cabinet, then performing a pre-set mix and timing strategy prior to addition into the production vessel. This becomes challenging to perform at a manufacturing scale when a 5-20% transfection complex volume range for a 1000L reactor translates to a 50-200L transfection cocktail. Completing a transfection operation at this scale is possible through batching, but it is a physically demanding operation leading to increased risk for errors. The Forge team has advanced the transfection unit operation to be an equipment-driven and system-controlled process, reducing the risk of operator manipulation, and has shown success and consistency.
Figure 3: Achieving Consistent Transfection Efficiency across Scale through Method Optimization
.jpg?width=800&height=378&name=Acheiving%20Consistent%20Transfection%20Efficiency%20across%20Scale%20through%20Method%20Optimization%20(Without%20Borders).jpg)
Figure 3: Achieving Consistent Transfection Efficiency Across Scale through Method Optimization. Ignition Cells were cultured in 50L SUBs, 500L SUB, and a 1L shake flask using equivalent expansion strategies. Each bioreactor or shake flask was transfected by either the research-based approach (control) or Forge’s optimized transfection method. The product was harvested at the same duration post-trans- fection. Clarified Lysate samples were pulled and submitted to compare titer (vg/mL) by a GOI-specific ddPCR method. Titer results were normalized to the shake flask control.
Following detailed planning and development, shake-down, and engineering runs are essential to evaluate platform models. These models account for scale-dependent factors and deploy scale-specific control strategies. Maintaining a consistent growth as well as metabolite profile, as depicted in Figure 4, is crucial for reliable manufacturing and is a primary metric for gauging upstream success.
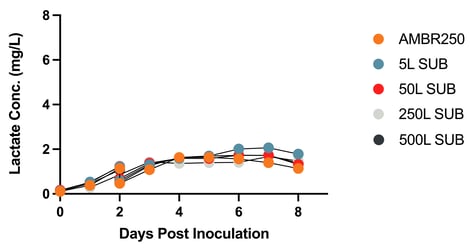
Figure 4: Metabolites Over Time at Increasing Bioreactor Scales
Glucose Concentration Across Bioreactor Scales Lactate Concentration Across Bioreactor Scales
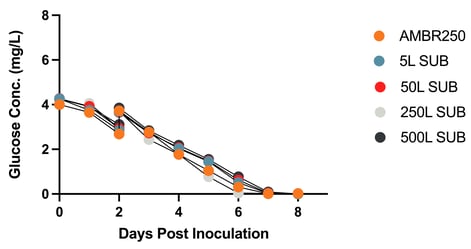
Figure 4: Metabolites Over Time at Increasing Bioreactor Scales. Cell cultures were seeded in the following bioreactors: Ambr250, 5L SUB, 50L SUB, 250L SUB, and 500L SU2B5. Each batch was diluted two days following inoculation and then transfected once the target viable cell density was reached. Samples were taken every 24 hours for eight days to measure glucose and lactate concentrations.

Conclusion:
Forge Biologics has developed a scalable and commercially viable platform process aimed at meeting the technical and capacity demand for AAV manufacturing. A well-defined, readily scalable upstream process was created by dedicating significant resources to process characterization through carefully designed studies focused on maintaining optimal mass transfer, managing shear stress, and ensuring homogeneity. Forge’s team of experts has fine-tuned bioreactor operations through meticulous optimization of parameters such as mixing, aeration, and essential process parameter control strategies. By leveraging these principles, Forge has effectively bridged the gap between small-scale laboratory productions and large-scale commercial manufacturing, thus establishing a pathway for streamlined and dependable AAV production.
Data-driven decision-making and robust process characterization will continue to propel our efforts toward an industry-leading 5,000L scale AAV manufacturing campaign. Scaling to new heights, Forge will continue to lead the way with paradigm-shifting innovation for the gene therapy community and, most importantly, patients.
References:
-
Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59-74. doi:10.1099/0022- 1317-36-1-59. PMID: 886304.
-
Seidel S, Maschke RW, Werner S, Jossen V, Eibl D. Oxygen mass transfer in biopharmaceutical processes: Numerical and experimental approaches. Chemie-Ingenieur-Technik. 2021;93(1–2):42-61. doi: 10.1002/cite.202000179
-
Harcum SW, Elliot KS, Skelton BA, et al. PID controls: the forgotten bioprocess parameters. Discov Chem Eng. 2022;2(1):1-18. doi: 10.1007/s43938-022-00008-z.
-
Fu Q, Polanco A, Lee Y S, Yoon S. Critical challenges and advances in recombinant adeno-associated virus (rAAV) biomanufacturing. Biotechnology and Bioengineering.2023;120(9):2601–2621. doi: 10.1002/bit.28412. PMID:37126355.